Coronavirus: What is Nucleic Acid Test (NAT) for COVID-19

What is Nucleic Acid Test (NAT)
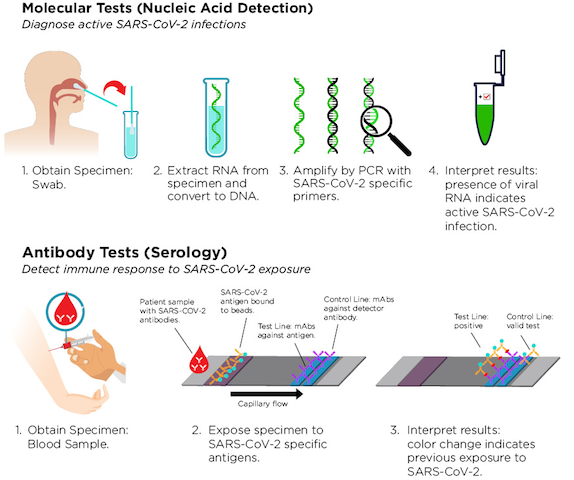
A nucleic acid test (NAT) focuses mainly on a particular nucleic acid to detect and identify a pathogen, mostly a virus or bacteria in human samples. The difference between this testing method and the rest is its ability to detect genetic components such as (DNA or RNA). Since antibodies require time to be noticed in the bloodstream early detection in the genetic materials, allow early diagnosis of patients, thus preventing further complications and fatality to COVD-19 patients.
The Difference of NAT to Antibody Test
A significant advantage of the nucleic acid test and specifically the nucleic acid amplification tests (NAATs), is its ability to amplify and make copies of the genetic material. Genetic sample materials collected for the testing is usually microscopic and not sufficient for extensive testing.
Nucleic acid amplification tests (NAATs) are being employed in clinical and medical laboratories in the mass testing phase of COVID-19 and applies several ways of amplifying genetic matter like:
- polymerase chain reaction (PCR),
- Strand displacement assay (SDA),
- Transcription mediated assay (TMA).
The polymerase chain reaction method is used to rapidly amplify the copies of DNA samples in a series of cycles of temperature changes to the tune of millions and billions enabling scientists to use microscopic DNA samples for study and testing. The repeated exposure of reactants to heating and cooling to create different temperatures (each cycle has two or three different temperature sets) produces different results for DNA melting and multiplication. Polymerase chain reaction testing involves the use of reagents, mainly primers and DNA polymerase. First, the DNA strands get separated at high temperatures through Nucleic acid denaturation and later the temperature is decreased, and the primers can bind to the complementary sequences of DNA. The generated DNA is then multiplied, creating a chain reaction leading to the amplification of the original DNA to exponential levels.
- Disposable Medical Face Masks with Elastic Ear Loop 3 Ply Breathable and Comfortable
- Disposal Protective Clothing for Medical Use
- N95/KN95 Protective Mask with Elastic Ear Loop
- Surgical Mask with Elastic Ear Loop 3 Ply Breathable and Comfortable
- ZeroVirus Space Portable Sterilization Bar
The use of polymerase chain reaction (PCR) technology is sensitive and robust. However, the tests are expensive and require highly trained personnel. The DNA strand displacement assay (amplification) comes to aid of nations that do not have sophisticated equipment nor the highly qualified medical staff in that the tests are carried out under isothermal conditions and therefore does not need expensive instruments. Strand displacement assay (amplification) is simple, fast, sensitive, specific, and moderate in terms of cost.
Strand displacement assay (amplification) has mostly been used as an alternative to the PCR in pathogen, cancers and hereditary diseases detention. Strand displacement assay (amplification) has the capabilities of providing visual and print results. Strand displacement amplification involves two primers recognizing two regions of the target sequence with one being a standard PCR primer and another SDA primers which bind to the bumper primers at the target sequences. The reaction mixture gets incubated at a temperature of 37C. Consequently, a primer triggered exponential reaction starts nicking, extension, and strand displacement. Additional primers accelerate amplification of the inner region of the target sequence with the response exhibiting a single and double nicking site cycle. To prevent false-positive results which may have significant medical, psychosocial, or medicolegal consequences culture confirmation is required for samples with COVID-19 positive results from populations since the predictive value of an assay is a function of the prevalence of the disease.
Transcription-mediated amplification (TMA) method uses a process in which temperature is maintained at a constant (does not change the nucleic acid temperature). The technique involves amplification of two enzyme RNA polymerase and reverse transcriptase, which makes copies of a strand of nucleic acid to be able to detect and test the sample matter. The rapid RNA and DNA amplification allow simultaneous detection of multiple pathogens in a single tube. Transcription-mediated augmentation has enabled clinical laboratories to perform nucleic acid tests for blood screening involving lesser procedures, time and faster results. Transcription-mediated amplification significant difference from PCR and ligase chain reaction is that it requires RNA transcription and DNA synthesis to produce an RNA amplicon from a target nucleic acid.
Transcription-mediated amplification has several advantages compared to other assay methods.
- Uses a water bath which is cheap and readily available instead of a thermal cycler
- Uses RNA amplicon than DNA amplicon decreasing carry-over contamination possibilities
Due to its high amplification in a shorter period (15–30 minutes), it is suited in makeshift laboratories involved in COVID-19.
When to Do NAT is Much Better
Nasopharynx, the space at the back of the nose, has the highest concentration of the COVID -19 virus. Nasopharyngeal (NP) swabs are ideal for sample collection and eventually, the molecular testing (Nucleic Acid Amplification Testing). However, it is difficult to obtain a substantial amount of the nasopharyngeal samples, which may lead to getting false-negative result in patients with the Coronavirus. The virus concentration in the nasopharynx changes throughout infection; thus, the timing of sample collection is essential. The perfect timing for collection of samples should be at the onset of a perceived infection to achieve the highest test sensitivity.
