Coronavirus: Must-know Knowledge of the New Coronavirus Vaccine
Since the first day of the outbreak of the new coronavirus epidemic, people have racked their brains to eliminate the virus. Unfortunately, the virus is still raging around the world and there are very few effective methods. The new coronavirus vaccine has been given the ultimate mission of conquering the virus.
As of September 18, the number of people infected with the new coronavirus has exceeded 30 million.
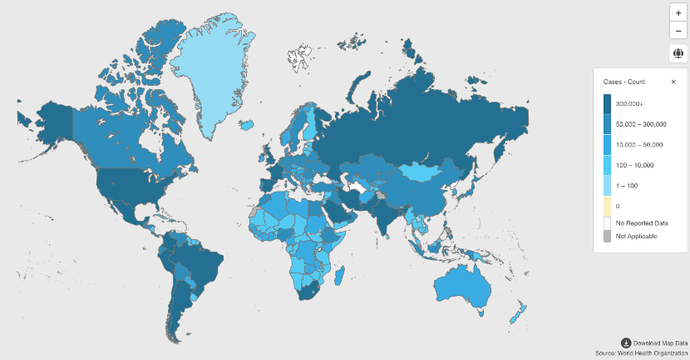
To this end, research institutions and pharmaceutical companies all over the world are racing to develop vaccines. According to data released by the WHO, as of September 2020, vaccines developed by many companies worldwide have entered the phase III clinical trial stage.
How many kinds of new crown vaccines are there? Is it really valid for life?
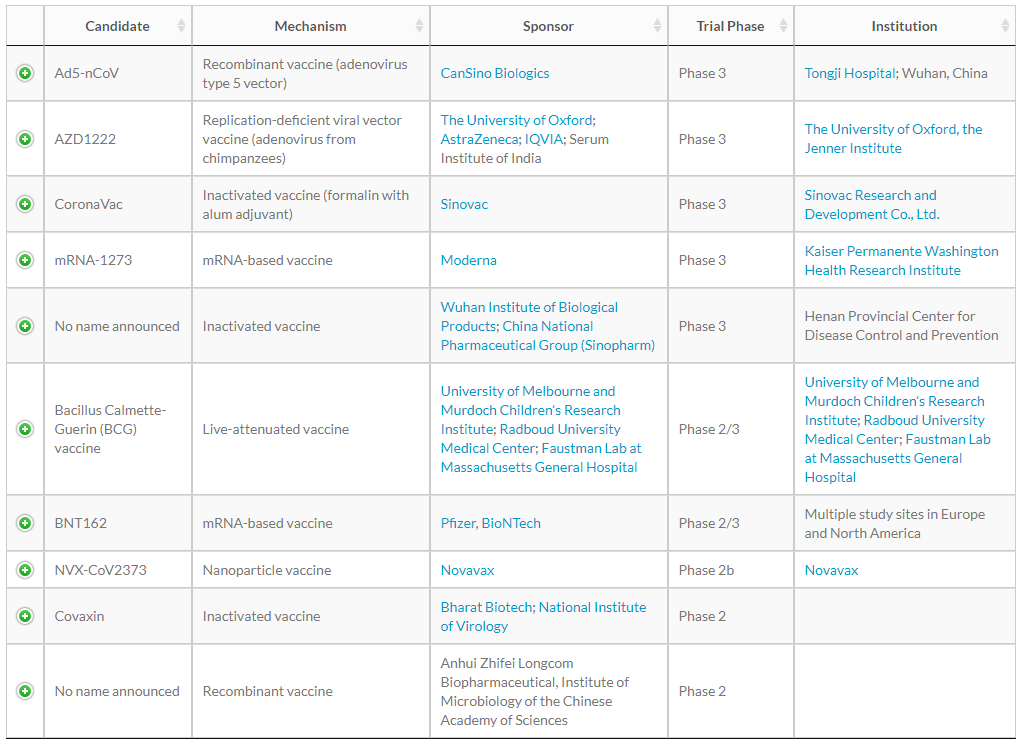
From the above table published by WHO, we can see that the types of vaccines developed by different countries and different research institutions are different. Currently, there are five main types of new coronavirus vaccines, including inactivated vaccines, nucleic acid vaccines, recombinant protein vaccines, non-replicating adenovirus vector vaccines, and attenuated influenza virus vector vaccines.
- Disposable Medical Face Masks with Elastic Ear Loop 3 Ply Breathable and Comfortable
- Disposal Protective Clothing for Medical Use
- N95/KN95 Protective Mask with Elastic Ear Loop
- Surgical Mask with Elastic Ear Loop 3 Ply Breathable and Comfortable
- ZeroVirus Space Portable Sterilization Bar
1. Inactivated vaccine
Inactivated vaccine is a classic vaccine preparation method. It is to cultivate the new coronavirus in vitro and then inactivate it to make it incapable of reproduction. However, the “corpse” of these viruses can still stimulate the body to produce antibodies and make immune cells remember the virus. appearance.
2. Nucleic acid vaccine
Nucleic acid vaccines include DNA vaccines and RNA vaccines. It is to wrap artificial chemically synthesized nucleic acid with nanoparticles and then inject it into the human body. After the nucleic acid vaccine enters the human body, it can produce the protein of the new coronavirus and stimulate the body to produce antibodies, thereby forming resistance to the new coronavirus.
3. Recombinant protein vaccine
The S protein is the key “key” for the new coronavirus to invade human cells. The S protein of the new coronavirus or the RBD region of the S protein responsible for binding to the receptor is produced by genetic engineering methods, and then injected into the human body to produce antibodies, which can prevent the new coronavirus from infecting the human body and resist the infection of the new coronavirus.
4. Non-replicating adenovirus vector vaccine
After the adenovirus is modified, the harmless adenovirus is used as a carrier, like a truck, carrying the S protein of the new coronavirus into the human body, stimulating the body to produce antibodies, and thus has the ability to resist the new coronavirus infection.
5. Attenuated influenza virus vector vaccine
Using an attenuated influenza virus vaccine that has been approved for marketing as a carrier, adding a new coronavirus protein can not only prevent new coronavirus infections, but also prevent influenza.
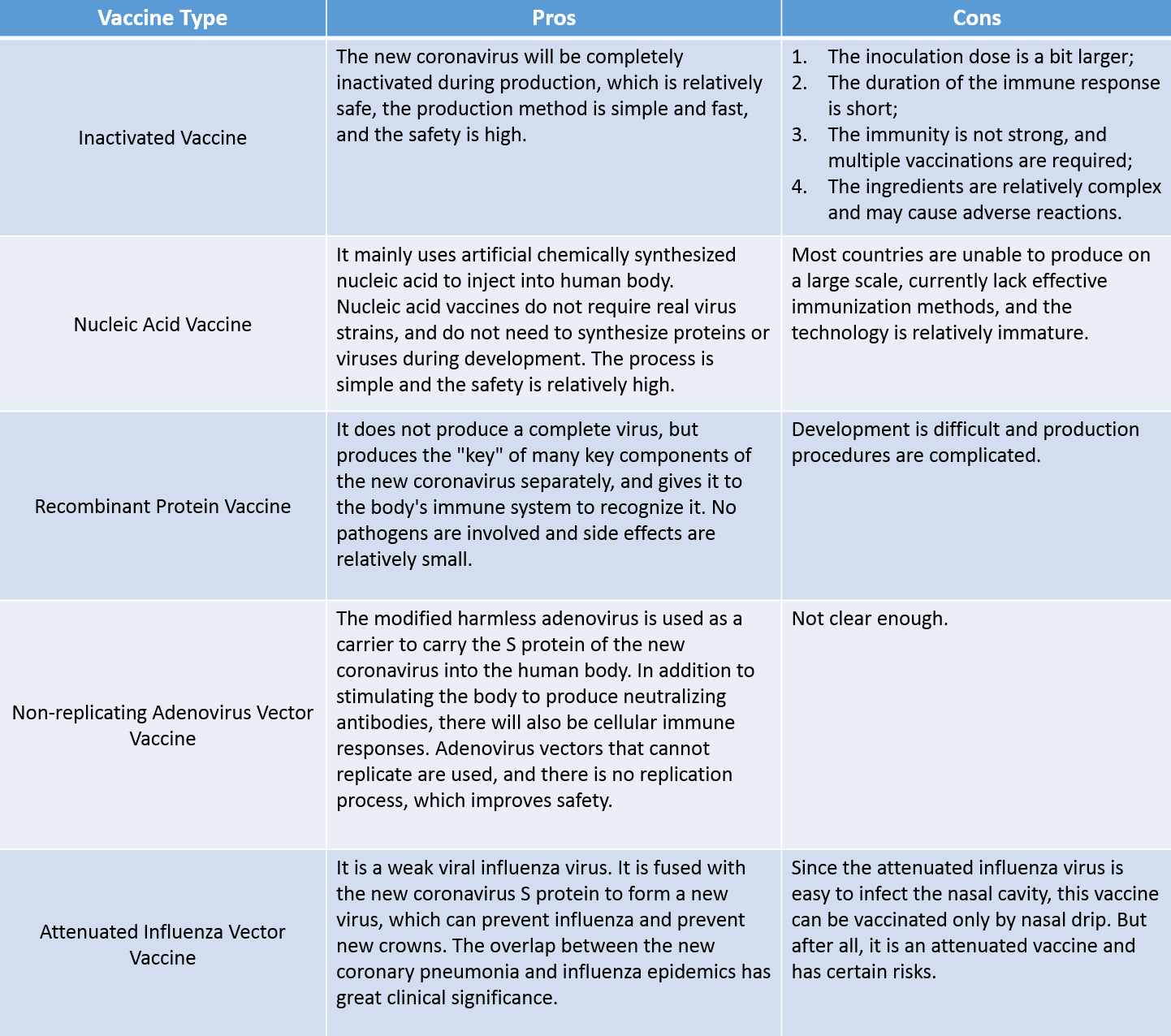
Advantages and disadvantages of different vaccines
Is the new coronavirus vaccine effective for life?
On August 25, after returning to Hong Kong from Europe, a survivor of COVID-19 tested positive again, marking his second infection with COVID-19. The antibodies produced by the patient during the first infection are not enough to prevent the second infection.
This case reminds us that the new crown vaccine may not provide lifelong protection. The protective effect of antibodies is not lifelong, it will decay, and the decay speed may be faster than expected. Wu Zunyou, the chief epidemiologist of the Chinese Center for Disease Control and Prevention, mentioned that for coronaviruses, antibodies produced after human infection can reach the protective effect in only about 6 to 12 months. How long can the protection of the vaccine last? At present, we can only speculate based on similar vaccines in the past. For example, the Ebola vaccine. After 6 months of the first shot, its immune response will decrease. After 6 months, the second shot will be strengthened. Valid for years. This can only be used as reference data.
After vaccination of different people, the length of protection will vary among individuals. Different types of vaccines take different time to work. It is generally believed that live attenuated vaccines will last longer than inactivated vaccines, but they have certain risks. Inactivated vaccines are simple to produce and relatively safe, but their action time is short.
Will the new coronavirus vaccine have a terrible ADE effect?
The ADE effect is an antibody-dependent enhancement response. Simply put, when the human body is infected by a virus, not only can the antibodies in the body not neutralize the virus, but it becomes a “Trojan horse” or “spy”, playing the opposite effect.
A paper in 2018 introduced the ADE effect of the inactivated SARS-CoV vaccine. The ADE effect assists the virus to enter the target cell, not only does not resist the virus, but increases the infection rate. This is a serious adverse reaction that can cause vaccine failure.
At the same time, when people are studying the Middle East Respiratory Syndrome (MERS) virus vaccine, it is found that when the antibody concentration is moderate, it is easy to promote the viral ADE effect. The new coronavirus, SARS and MERS are both coronaviruses, and whether the vaccine will have an ADE effect in clinical applications remains to be further studied. If there is an ADE effect, it may be necessary to pay attention to the subsequent design and development of vaccines. The titer of the antibody produced by the body must be high, otherwise it will produce obvious adverse reactions.
The new coronavirus is a single-stranded RNA virus. Due to the lack of a correction mechanism during replication, it is relatively easier to mutate. After the mutation of the new coronavirus, the vaccine developed and designed for the original virus structure may become invalid.
Conclusion:
Because the epidemic is urgent and the time for clinical trials is relatively short, the vaccine produced in an emergency has not undergone large-scale clinical trials and long-term observation. Many side effects or adverse effects of the vaccine are still unknown. However, there are no sufficient data on the effectiveness and durability of the vaccine. Whether the vaccine is used on a large scale, it is necessary to wait for more research data and the further development of the epidemic.
It should be emphasized that we do not have to wait for the success of vaccine development, the key is to do scientific protection:
- Pay attention to washing your hands frequently in your life, and don’t go to places where people gather;
- Frequently open windows for ventilation and disinfection work.
- Develop the habit of wearing a mask when going out.
- Exercise moderately, take in enough nutrition to ensure adequate resistance.
