Coronavirus: Remdesivir Does Not Work from WHO Study

CNN reported that the World Health Organization found in a clinical trial covering 11,000 patients in 30 countries that Remdesivir, a drug developed by the American pharmaceutical company Gilead, has no effect on reducing the death rate of new coronavirus pneumonia, and it has not shortened the recovery time of inpatients.
In addition, the World Health Organization has also tested three other drugs, namely hydroxychloroquine, a malaria treatment drug promoted by U.S. President Trump and conservative forces in the United States, and lopinavir-ritona, which is a combination therapy used to treat HIV, and interferon. The results are also unsatisfactory. No drug could effectively reduce the mortality of patients or shorten the recovery time.

- Disposable Medical Face Masks with Elastic Ear Loop 3 Ply Breathable and Comfortable
- Disposal Protective Clothing for Medical Use
- N95/KN95 Protective Mask with Elastic Ear Loop
- Surgical Mask with Elastic Ear Loop 3 Ply Breathable and Comfortable
- ZeroVirus Space Portable Sterilization Bar
However, CNN stated that these findings of the World Health Organization have not yet been peer reviewed by academia. This also gave Gilead, who developed Remdesivir, space to refute.
CNN said that Gilead expressed dissatisfaction with the results of the World Health Organization trial in a statement, saying that the results of the trial were compared with the results of other peer-reviewed trials that showed remdesivir can improve the recovery time of patients. Not consistent”. Gilead also believes that the World Health Organization’s data and experiments are not rigorous.
On Twitter, some American “patriotic netizens” who support Trump and the right-wing conservative forces in the United States have also expressed that they “do not believe in the conclusion of the World Health Organization, which is helping China.”
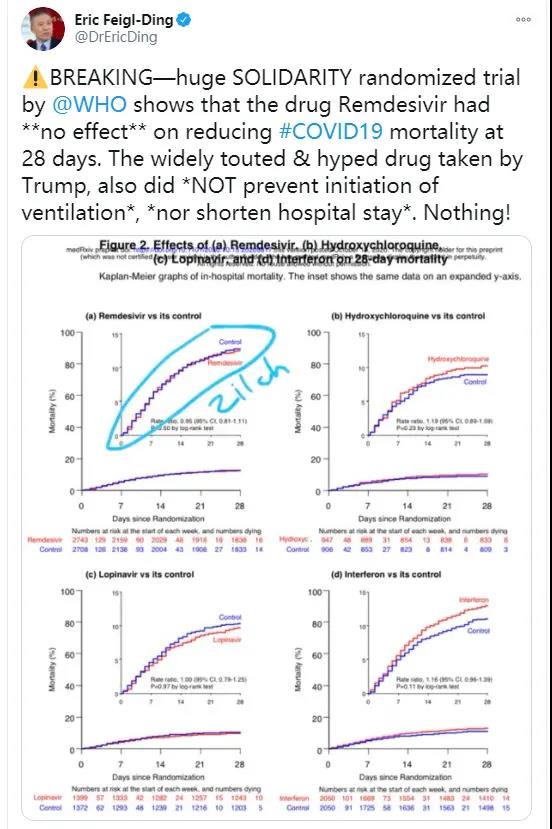
However, some American netizens who oppose Trump believe that the results of the World Health Organization’s test have pierced yet another piece of bogus Trump blew out.
Previously, a clinical trial in China that was suspended halfway showed that Remdesivir had no obvious effect in improving mortality and disease course, which is similar to the situation found by the World Health Organization. A large clinical trial of the drug conducted by an official US agency showed that Remdesivir can shorten the recovery time of severely ill patients, but it is not effective for mild patients.
It is worth mentioning that the US experiment was once aroused by the adjustment of the objectives of the experiment. According to media reports at the time, the initial goal of the trial was to test whether Remdesivir can reduce the mortality rate. However, the effect was not significant and it was replaced in order to shorten the recovery time of patients.
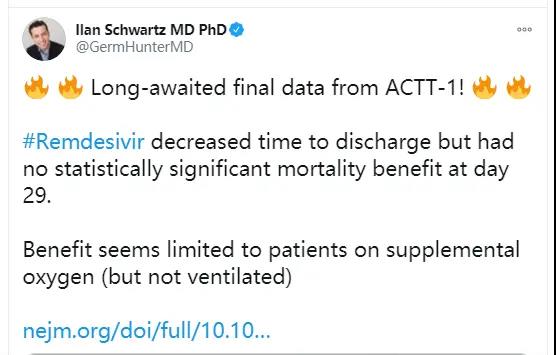
The results of a clinical trial of Remdesivir published in the well-known medical journal “New England Medicine” on October 8 seem to confirm the results of the United States, that is, Remdesivir is effective in improving hospitalized patients with new coronary pneumonia. It has a certain effect, but it is mainly for people who need oxygen but do not need to be on a ventilator. In reducing mortality, the drug failed to show statistical significance.
Finally, CNN said that the World Health Organization currently plans to start clinical trials on monoclonal antibodies such as the cocktail antibody therapy used by Trump after being infected with the new coronavirus not long ago.
